Project management
The performance, delivery time, and budget of the delivered system are guaranteed.
Commitment to the result
qmt is a provider of customized solutions for testing and quality control.
Each project begins with understanding the client's technical and organizational needs. Based on this understanding, we define the optimal solution, incorporating the necessary customizations to meet specific requirements. This approach ensures that we perfectly meet the client's expectations with a solution based on a proven standard.
Customization can range from adding a small software function to creating a product dedicated to a specific client. In all cases, QMT guarantees the functionality and performance of the delivered system.
Customizable solutions to perfectly meet your needs
measure
Tailor-made solutions designed to meet specific requirements
Tailor-made solutionsA multidisciplinary project team coordinated by a project manager
The QMT organization is customer-focused. A team is appointed at the start of each project and works within a structured project process that ensures the client's objectives are met in terms of schedule, quality, cost, and performance. Regular progress meetings are held throughout the project, from launch to final system validation at the client's site. This approach has enabled us to develop systems that meet demanding testing and quality control requirements in diverse fields such as watch component production, stainless steel tubing, and consumer goods.
Our services can cover the successive stages of a project, from the development of the specifications to the delivery and maintenance of the installation.
- A project manager (PM) who will be the client's primary contact throughout the project, is in charge of the organization and administration of the project.
- A technical leader who will be responsible for ensuring the technical solution meets the specifications and for supervising the technical team.
- A salesperson who will be in charge of business relations.
- A technical team with the necessary expertise for the project
QMT's offerings are based on client specifications. QMT supplements these specifications with a "Response to Requirements (RR)." This document commits QMT to achieving the proposed performance within the defined limits. To maximize efficiency, we propose developing the specifications collaboratively with the client in the form of a sprint.
We are active in all types of industries , but have particular expertise in the medical, watchmaking, rail, and automotive industries, as well as in special machinery. Our list of references testifies to our successes.
Some projects require an initial phase of theoretical or experimental study to validate their feasibility. Our team has a laboratory equipped to experimentally resolve technical doubts. In addition, the team includes scientists who can address the problems theoretically.
QMT has a project management information system (planning, cost management, etc.) to effectively manage projects. The QMT Link collaborative system structures all tasks and documentation; this system can be made accessible to clients to guarantee efficient communication, traceability, and transparency.
The basic project process is structured in a "V" model and is suitable for projects with specifications defined at the outset. We offer an alternative with an Agile process for projects whose objectives and priorities may evolve during execution. In both cases, to ensure project success, a project manager is responsible for the smooth running of the projects. Continuous monitoring guarantees adherence to deadlines and budgets.
The internal and client kick-off sessions aim to ensure all stakeholders are aligned from the moment the order is received. The project is planned within the internal project management system, and development tasks are monitored at least once a week. This phase begins with the initial design review ("we say what we're going to do") and ends with internal validation ("we did what we said we would do"), after the system has been tested by the development team.
A specific organizational structure is implemented throughout the project to carry out the validations. This includes protocol writing, internal validation, client validation at QMT premises (FAT), and finally, on-site validation at the client's location (SAT). We are proficient in statistical tools for both metrological and attribute-based validations (R&R capabilities).
The requirements of ISO 13485 apply to companies that design control systems for medical devices. Throughout the value chain, QMT products are designed, developed, and manufactured with the aim of being safer and more effective. Particular emphasis is placed on traceability and risk analysis for the medical sector. The risk analysis is integrated into the product's risk management documentation.
Customized solutions for testing and quality control
Mechatronics for value-added measurements
Our system architects design mechatronic systems by defining multi-disciplinary functions that meet client requirements. They have the support of experts in each technical field to propose the best solutions and possess proven experience in quality control, testing, and mechatronic design, enabling them to fully understand client needs and expert constraints.
Quality control and testing applications often require mechatronic solutions that combine mechanical, electrotechnical, electronic, optical, acoustic, and multi-physics components with software. Our mechatronic system architects have a deep understanding of the various disciplines involved and are experts in some of them. Furthermore, they have years of experience in quality control and testing. This experience allows them to understand the client's needs, refine them, define the system with the team, and understand its constraints. They are the guarantors of the performance of our turnkey solutions.
Each QMT architect brings their own unique perspective and personal experience to deliver the best solution. However, mastering certain methods and tools is essential to ensure the sustainability of this expertise. The Design Document (DD) serves as our guiding thread throughout the development and lifecycle management of our products. It outlines the client's requirements and acceptance criteria, as well as our responses and constraints to these requirements. The team, technically led by the architect, documents its functional analysis and functional diagrams within the DD, using various levels of abstraction necessary for understanding the system and its implemented functions. The functions and technical choices are also documented in the DD.
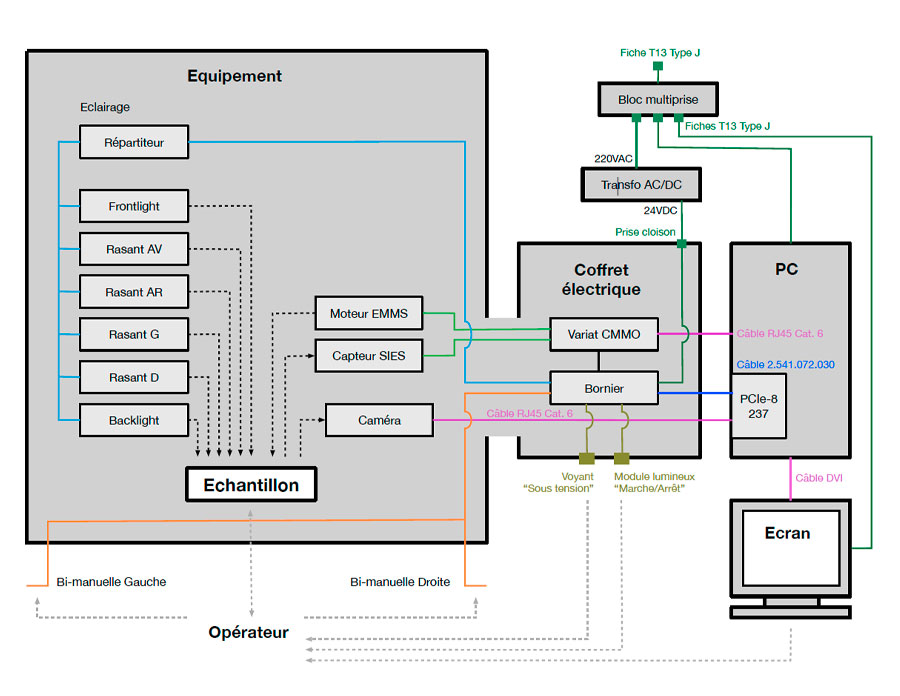
Other tools, some of which are made available to our clients, complement our portfolio of architectures. Failure Mode, Effects, and Criticality Analysis (FMECA) is an example of a powerful tool that helps us better design our systems.
An iteration of FMEA (Failure Mode and Effects Analysis)

QMT's expertise in implementing solutions
Understanding the client's needs, defining the optimal solution architecture, and integrating the system into the client's environment.
Software development or customizations integrating signal acquisition, processing, communication, and results management
Design and development of Multi-Inspection Mechatronic (M2IS) systems for testing and micromanipulating devices to be controlled
Development and design of innovative optical systems and algorithms to reproduce human vision (3D vision, augmented stereoscopic vision, etc.)
Integration of multi-physics measurements (torque, force, velocity, position, etc.) into systems to enable characterization and automation
Model selection, dataset creation, data management, and integration into an automated process