The medical industry
Testing and quality control solutions carried out in compliance with the ISO13485 standard
Testing and quality control of medical devices
qmt is a specialist in the creation of testing and quality control systems. For more than 20 years, we have been producing this type of solution for medical applications in Switzerland and France. We have put in place an organization particularly suited to the needs of this industry. It includes rigorous project management, risk management, design procedures taking into account the system architecture.
- The management system certified ISO 9001 and ISO 13485
- Project management and architecture
- Project process and risk management according to FMEA
- A medical standard for IQ / OQ / PQ qualifications
Customizable solutions to perfectly meet your needs
measure
Tailor-made solutions designed to meet specific requirements
Tailor-made solutions
Commitment to results
The qmt organization is very “customer satisfaction” oriented. A project team is appointed at the start of development, it works in accordance with the project process which guarantees the achievement of the client's objectives in terms of planning, quality, costs and performance.
QMT projects are based on customer specifications (URS). qmt completes the specifications with a “Response to Needs (RB)”. This document commits qmt to achieving the proposed performances within the defined limits.

qmt offers the definition of requirements in collaborative mode
Defining requirements for a new test or quality control system is often a bottleneck for project initiation. It represents an effort and a delay for the customer but also for the supplier who must respond to the requirements.
qmt offers the definition of requirements collaboratively in the form of a sprint to address the presentation of the context and issues, the expression of needs, the definition of constraints, the formalization of requirements, the FMEA risk analysis and the validation of specifications with the customer. During this sprint, the validation methods are also defined to integrate the validation constraints in the design and production phase. is also initiated. The deliverables of this phase are:
- Horned beast
- Functional analysis
- Specification in the form of response to needs
- FMEA risk analysis according to ISO14971
- Validation protocol according to current standards (ISO13485, CFR21Part11, IEC 60601-1-11, etc.)
More than 100 customers have trusted qmt
- Affolter Technologies Malleray - Switzerland
- Audemars Piguet Le Brassus - Switzerland - testimonial
- Atokalpa Alle - Switzerland
- BONINCHI SA Meyrin - Switzerland
- CLA Clinical Laboratory Automation SA Delémont - Switzerland
- DUBOIS DEPRAZ Le Lieu - Switzerland
- IWC Schaffhausen Schaffhausen - Switzerland
- Jaeger-LeCoultre Le Sentier - Switzerland
- Jean Singer La Chaux-de-Fonds - Switzerland
- KIF Parechoc Le Sentier - Switzerland
- LA PIERRETTE Le Brassus - Switzerland
- Metalor Neuchâtel - Switzerland
- OMEGA Bienne - Switzerland - testimonial
- PANERAI Neuchâtel - Switzerland
- PATEK PHILIPPE Geneva - Switzerland
- Piaget Geneva - Switzerland
- SVM Micromechanics Villeret - Switzerland
- Swatch Group (various industrial companies) Switzerland, France and Thailand
- Valfleurier Buttes - Switzerland
- VOH Courtelary - Switzerland
- Baud Industries France, USA, Poland and Mexico - testimonial
- Borg Warner Eyrein - France
- Bontaz-Centre Marnaz - France
- CGR PMPC Besançon - France - testimony
- CODEC SA Dombresson - Switzerland
- Continental Automotive Switzerland Rüthi - Switzerland
- Demidec Marignier - France
- DOW Europe Horgen - Switzerland
- DuPont de Nemours International Geneva - Switzerland
- EFI Automotive
- Federal Mogul
- G. Cartier Technologies Cluses - France
- HID Global Switzerland Granges (Veveyse) - Switzerland
- HPO Scientrier Group , Cluses - France - testimonial
- Joseph Martin Vougy - France - testimony
- Kartesis Marignier - France
- Kuk Electronik Appenzell - Switzerland
- LEM Geneva - Switzerland - testimony
- Minesco - France
- NTN-SNR Annecy - France
- Pernat Emile Marignier - France - testimony
- POLYDEC Bienne - Switzerland - testimony
- Poppe + Potthoff Scionzier, Bonneville - France - testimony
- Raymond Dubosson Cluses - France - testimony
- Robert Bosch Automotive Rodez - France
- SONCEBOZ Sonceboz - Switzerland
- Valeo
- CFF SBB Bern - Switzerland
- CIMES Valenciennes - France
- Hitachi Energy Geneva - Switzerland
- Sécheron SA Geneva - Switzerland - testimonial
- Speno International Geneva - Switzerland - testimonial
- Aleva Neurotherapeutics SA Ecublens - Switzerland
- Allergan Medical Ecublens - Switzerland
- Becton Dickinson Medical Le Pont de Claix - France
- Cytiva SA Nyon - Switzerland
- Flextronics Solothurn - Switzerland
- Forteq Nidau Nidau - Switzerland
- Hamilton Company Reno - USA
- Heraeus Materials Yverdon-les-Bains - Switzerland
- Johnson & Johnson Le Locle - Switzerland
- IMA Medtech La Chaux-de-Fonds - Switzerland
- Kyphon Medtronic Galway - Ireland
- Maillefer Instruments Ballaigues - Switzerland
- Medtronic Switzerland, Puerto Rico, Singapore
- Nolado Treff Degersheim - Switzerland
- Nypro Healthcare Knittlingen - Germany
- Suturex & Renodex Sarlat - France
- Zubler AG Uzwil - Switzerland
- Bobst Prilly - Switzerland
- BTG Eclepens Eclepens - Switzerland
- Ebnat Kappel Ebnat - Switzerland
- Europipe Dunkirk - France
- Firmenich Geneva - Switzerland
- HID Global Switzerland SA Granges (Veveyse) - Switzerland
- Jokey - Germany, France, Canada, Belarus
- LN Industries Champagne - Switzerland
- Metalor Technologies Courville-sur-Eure - France
- Nestlé Lausanne - Switzerland
- Novartis Basel - Switzerland
- Philip Morris Lausanne and Neuchâtel - Switzerland
- Profalux Thyez - France - testimonial
- Rollvis Geneva - Switzerland
- Somfy
- STMicroelectronics
- Staubli

qmt offers the achievement of medical qualifications
The validation effort in the medical field is high and represents a significant completion time which can compromise the success of the project.
Basically, qmt carries out the FAT and SAT according to the client's validation protocol to facilitate final qualification by the client (IQ, OQ and PQ), qmt also provides the system's documentary package. QMT support can continue after the SAT with the provision of a resource to support the customer during the qualification of the equipment
qmt offers a 2nd qualification mode with parallelization of the efforts of qmt and the client to reduce the implementation time. qmt can also take responsibility for this qualification with a resource seconded to the client's premises.
QMT's expertise in implementing solutions
Understanding the client's needs, defining the optimal solution architecture, and integrating the system into the client's environment.
Software development or customizations integrating signal acquisition, processing, communication, and results management
Design and development of Multi-Inspection Mechatronic (M2IS) systems for testing and micromanipulating devices to be controlled
Development and design of innovative optical systems and algorithms to reproduce human vision (3D vision, augmented stereoscopic vision, etc.)
Integration of multi-physics measurements (torque, force, velocity, position, etc.) into systems to enable characterization and automation
Model selection, dataset creation, data management, and integration into an automated process
Example of a medical measuring station
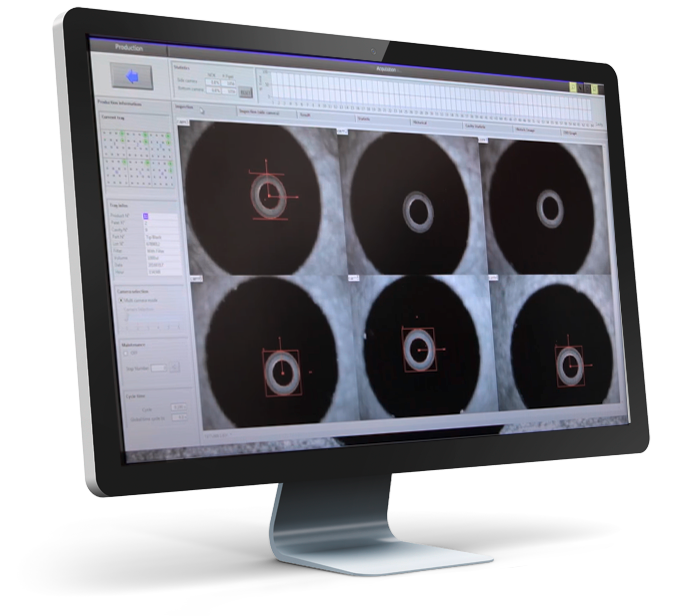
Example of custom equipment
